Which of the following chemical formulas correctly represents 2 molecules of silver bromide? 2AgBr C. Ag2Br2 Ag(Br)2 D. 2Ag2Br2 6. What is the What is the correct formula for the compound lithium oxide? 9. The compound formed from the elements calcium and chlorine is known as Chlorine...Which pair of elements is most likely to form an ionic compound with each other barium, chlorine calcium, sodium oxygen, fluorine sulfur Which of the following compounds would you expect to be ionic? Predict the formula of the ionic compound that forms from magnesium (+1) and oxygen (2Which are likely to be molecular? (a) SiCl4 -- Molecular(b) LiF-- Ionic(c) BaCl2-- Ionic(d) B2H6 -- Molecular(e) KCl-- Ionic(f) C2H4-- Molecular(Reference: Chang 2.49) 9.What is the l.CuSO4⋅ 5H2O - Copper (II) Sulphate Monohydrate 11. Write the formulas for the following compounds: (18 points) 12.4. Which of the following elements is most likely to form a molecule that does not obey the octet rule? 11. Which of the following compounds would be expected to have the strongest ionic bonds?Arrange the following compounds in order of increasing solubility in water: * O2 * LiCl * Br2 * CH3OH Like dissolves like; that is, polar compounds usually are soluble in water A.Li and S B.O and S C.Al and O d.F and Cl e.I and K F.H and N Which of the following pairs of elements will not form ionic.
Ionic Compounds Test Flashcards | Quizlet
Ions and Ionic Compounds. Elements combine in a specific ratio to form compounds. A cation results from an element losing an electron (e-) to form a positively charged species. The magnitude of the charge of the cation depends on the number of electrons lost.11) Which of the following compounds would you expect to be ionic? The charge of 13) Which pair of elements is most likely to form an ionic the ion is -1. compound with each other?13. Which of these pairs of elements would be most likely to form an ionic compound? Since each double bond corresponds to a pair of electrons, the carbon atom can form only 2 double bonds. 33. Which of the following molecules contains both ionic and covalent bonds?The formula for an ionic compound follows several conventions. First, the cation is written before the anion. Because most metals form cations and (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) This is a clue that the other part of the formula...

47 8 Which of the following compounds are likely to be ionic Which...
Name each of the following compounds, and tell wh… Write appropriate formulas for the ionic compounds you expect to form, and give the name of each. (a) chlorine and bromine (b) phosphorus and bromine (c) lithium and sulfur (d) indium and oxygen (e) sodium and argon (f) sulfur and bromine...When searching for pairs of elements that will form an ionic bond, one must be a metal and one must be a nonmetal. Importantly, noble gases, although nonmetals, do not form ionic bonds.An ionic compound is one in which at least two of the elements or compounds in the group are oppositely-charged ions held together in an ionic bond. In the formation of this bond, a metal atom, either alone or bonded in a molecule, donates an ion to a nonmetallic atom.The chart below shows monatomic ions formed when an atom loses or gains one or more electrons, and the ionic compounds they form. You can check your periodic table to see that the cations are monatomic ions formed from metals, and the anions are monatomic ions formed from nonmetals.Which of the following pairs of elements and valence electrons is incorrect? Which of the following does not have a noble gas electron configuration? (or Which of the following is not (c) four single bonds around the central carbon atom. (d) two equivalent resonance forms.
Give it up Brooke. And by way of the manner, there are no 100% ionic compounds.
This is a tough thought as a result of there isn't a clear distinction between ionic and covalent bonds and between ionic and covalent compounds. "Ionic" and "covalent" exist on a continuum. Most bonds have characteristics of each ionic and covalent bonds.
In truth there are NO A hundred % ionic compounds. Even the bonds in CsF which have the greatest electronegativity distinction (DEN) are Eight percent covalent.
More chemical bonds are covalent than are ionic. All bonds like alongside a continuum between ionic and covalent and feature some traits of each. We make a selection an arbitrary point (the place the electronegativity distinction is above 2.0) to say that a bond is predominately ionic.
It is imaginable to compute the % ionic persona in a bond with the following method:
percent ionic personality = 100(1 - e^(-DEN^2/4) )
There are many people who nonetheless do not needless to say bonding can't be reduced to both "ionic" or "covalent. Keep in mind that there are NO compounds which are completely ionic. Actual chemical bonds lie along a continuum between covalent and ionic. Even the most ionic of bonds (Cs-F) is not completely ionic (it is 8 percent covalent) and share electrons to a certain degree.
Therefore, it really doesn't make sense to try to peg a bond as either "ionic" or "covalent". What does make sense is to try to place the bond somewhere along the continuum. The key is to look at the electronegativity differences. The greater the electronegativity difference, the greater the percent ionic character.
For instance, in benzoic acid there are C-O bonds which are quite covalent and O-H bonds which are also very covalent, as well as C-H bonds which are almost 100 percent covalent.
In KCl the bonds are very polar covalent. The electronegativity difference is (3.16-0.82 = 2.34) great enough to say that the bonds behave as if they were ionic (yet the percent ionic character is 75 percent ionic, or 25 percent covalent).
Sodium sulfate also has a high percent ionic character, while sucrose has bonds which are much more covalent in nature (it has the same bonds as does benzoic acid).
Sometimes people think that if they dissolve the compound in water and the solution conducts electricity that the compound will be ionic. This only works part of the time. Lots of molecular compounds with covalent bonds will dissolve in water to produce ions which will make the solution conduct electricity. So this turns out to be an ineffective test for ionic vs covalent compounds. The best example of why this doesn't work in hydrochloric acid. Hydrochloric acid is produced with molecular HCl gas, with covalent bonds, dissolves in water to produce hydrogen ions and chloride ions. There are many other compounds which will produce ions in solution, but which have predominately covalent bonds.
A better test that involves conductivity is to melt the compound. If the molten substance conducts electricity then there is a greater likelihood that the compound contains ions, and has ionic bonds.
In fact, the oxidation state of an element will determine how ionic or covalent the bonds are. Consider permanganate ion, MnO4-. In permanganate the bonds are much more covalent than the bonds between Mn and Cl in MnCl2 even though oxygen is more electronegative than chlorine.
Sometimes people will try to use melting point as an indicator of ionic or covalent bonds. The melting point is determined by intermolecular forces, not the type of bonding within the molecule. In fact the substance with the highest melting point, diamond, is composed of purely covalent bonds.
The final analysis is that the kind of bonding is not as clear minimize as some teachers (who don't seem to be chemists) check out to make it out to be. To resolve the predominate kind of bonding you in point of fact need to have a look at a variety of factors including dipole second, bond power, electronegativity distinction, and whether or not the compound bureaucracy discrete molecules or exists as a community forged.
Solved: 2. Which Of The Following Pairs Of Elements And Va ...

Sodium oxide combines violently with water. W... | Clutch Prep

Chemistry 11

4.2 Predict if a compound of 2 elements is ionic using the ...

Chemistry Archive | July 26, 2012 | Chegg.com

Chemistry Archive | March 03, 2017 | Chegg.com
inorganic chemistry - Shapes of ionic compound - Chemistry ...

Which of the following pairs of elements would most likely ...

What is the correct formula for the product of the ...

Covalent Bond Worksheet | Homeschooldressage.com

CH150: Chapter 3 - Ions and Ionic Compounds - Chemistry

chem.docx - Question 1 Which one of the following(pure ...

SOLVED:Which of the following pairs of elements a…

Which pair of elements below would be least likely to form ...

Answer: Which of the following contains BO... | Clutch Prep

chem.docx - Question 1 Which one of the following(pure ...

7- Chapter 9 Questions

Which one of the following elements is most likely to form ...

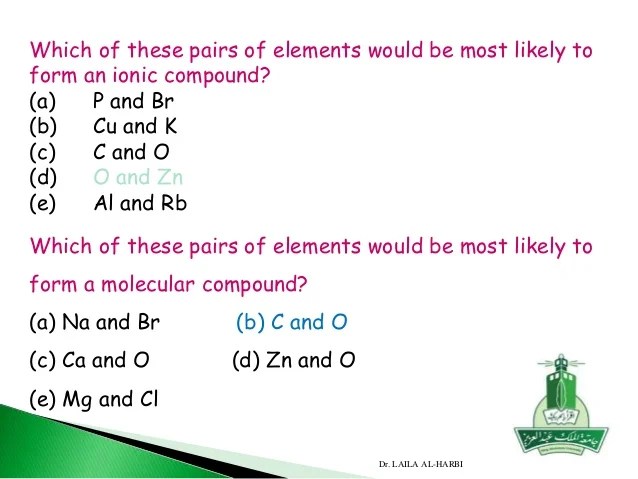
Which of these pairs of elements would be most likely to ...

Which of the following pairs of elements would be most ...

Chapter 2
0 Comment to "Which Of The Following Pairs Of Elements Is Most Likely To Form An..."
Post a Comment